Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Ito, Hiroto*; Shiotsu, Hiroyuki; Tanaka, Yoichi*; Nishihara, Satomichi*; Sugiyama, Tomoyuki; Maruyama, Yu
JAEA-Data/Code 2018-012, 42 Pages, 2018/10
Chemical composition of fission products transported in nuclear facilities in severe accidents is controlled by slower chemical reaction rates, therefore, it could be different from that evaluated on the chemical equilibrium assumption. Hence, it is necessary to evaluate the chemical composition with reaction kinetics. On the other hand, databases applicable to the analysis of nuclear facilities have not been constructed because knowledge of reaction rates of complex chemical reactions in severe accidents is currently limited. Accordingly, we have developed the CHEMKEq code based on a partial mixed model with chemical equilibrium and reaction kinetics to decrease uncertainties of the chemical composition caused by the reaction rate. The CHEMKEq code, under mass conservation law, firstly evaluates chemical species obeying the chemical equilibrium model, and then, relatively slow reactions are solved by the reaction kinetics model. Moreover, the CHEMKEq code has a multiplicity of use in evaluations of chemical composition because general chemical equilibrium and reaction kinetics models are also available and databases required to calculation are external file formats. This report is the user's guide of the CHEMKEq code, showing models, solution methods, structure of the code and calculation examples. And information to run the CHEMKEq code is summarized in appendixes.
 -ZrO
-ZrO pseudo-binary phase diagram
pseudo-binary phase diagramAlbiol, T.*; Serizawa, Hiroyuki; Arai, Yasuo
Journal of Nuclear Science and Technology, 39(Suppl.3), p.834 - 837, 2002/11
no abstracts in English
Kobayashi, Kaoru*; Kaminaga, Masanori; Haga, Katsuhiro; Kinoshita, Hidetaka; Aso, Tomokazu; Teshigawara, Makoto; Hino, Ryutaro
JAERI-Tech 2002-005, 118 Pages, 2002/02
In order to examine the radiation safety of a spallation mercury target system, it is necessary to clarify the chemical forms of spallation products generated by spallation reaction with proton beam. As for the chemical forms of spallation products in mercury that involves large amounts of spallation products, these forms were estimated by using the binary phase diagrams and the thermochemical equilibrium calculation based on the amounts of spallation product. Calculation results showed that the mercury would dissolve Al, As, B, Be, Bi, C, Co, Cr, Fe, Ga, Ge, Ir, Mo, Nb, Os, Re, Ru, Sb, Si, Ta, Tc, V and W in the element state, and Ag, Au, Ba, Br, Ca, Cd, Ce, Cl, Cs, Cu, Dy, Er, Eu, F, Gd, Hf, Ho, I, In, K, La, Li, Lu, Mg, Mn, Na, Nd, Ni, O, Pb, Pd, Pr, Pt, Rb, Rh, S, Sc, Se, Sm, Sn, Sr, Tb, Te, Ti, Tl, Tm, Y, Yb, Zn and Zr in the form of inorganic mercury compounds.
Savage, D.*; Lemke, K.*; Sasamoto, Hiroshi; Shibata, Masahiro; Arthur, R. C,*; Yui, Mikazu
JNC TN8400 2000-004, 30 Pages, 2000/01
Modeling approaches that have been proposed for cement-water system are reviewed in this report, and relevant supporting thsrmodynamic data are compiled. The thermodynamic data include standard molal thermodynamic properties of minerals and related compounds comprising cements, and equilibrium constants for associated hydrolysis reactions. Similar data for minerals that are stable in hyperalkaline geologic environments (e.g., zeolites) are also included because these minerals could be formed as hyperalkaline fluids emanating from cementitious matelials in a repository for radioactive wastes interact with the surrounding host rock. Standard molal properties (i.e., standard molal Gibbs free energies and enthalpies of formation and standard molal entropies), and/or equilibrium constants for associated hydrolysis reactions, are included for. (1)cement minerals and related compounds (Reardon, 1992; Glasser et al., 1999) (2)calcium-silicate hydrate minerals (Sarkar et al., 1982), and (3)zeolites (calorimetric and estimated values from various sources) All these data are accepted at face value, and it is therefore cautioned that the data, considered as a whole, may not be internally consistent. It is also important to note that the accuracy of these data have not been evaluated in the present study. Several models appropriate for cement-water systems have been proposed in recent years. Most are similar in the sense that they represent empirical fits to laboratory data for the CSH gel-water system, and therefore not thermodynamically defensible. An alternative modeling approach based on thermodynamic principles of solid-solution behavior appropriate for CSH gel has recently been proposed, however. It is reviewed in the present study, and evaluated in relation to experimental results obtained by JNC on cement-water interactions. The solid-solution model is based upon a thermodynamically- and structually-justifiable description of CSH gel in terms of a non-ideal ...
Yui, Mikazu; ; Shibata, Masahiro
JNC TN8400 99-070, 106 Pages, 1999/11
This report is a summary of status, frozen datasets, and future tasks of the JNC thermodynamic database (JNC-TDB) for assessing performance of high-level radioactive waste in geological environments. The JNC-TDB development was carried out after the first progress report on geological disposal research in Japan (H3). In the development, thermodynamic data (equilibrium constants at 25  C, I=0) for important radioactive elements were selected/determined based on original experimental data using different models (e.g., SIT, Pitzer). As a result, the reliability and traceability of the data for most of the important elements were improved over those of the PNC-TDB used in H-3 report. For detailed information of data analysis and selections for each element, see the JNC technical reports listed in this document.
C, I=0) for important radioactive elements were selected/determined based on original experimental data using different models (e.g., SIT, Pitzer). As a result, the reliability and traceability of the data for most of the important elements were improved over those of the PNC-TDB used in H-3 report. For detailed information of data analysis and selections for each element, see the JNC technical reports listed in this document.
Sasamoto, Hiroshi; Yui, Mikazu; Arthur, R. C,*
JNC TN8400 99-033, 153 Pages, 1999/07
The results of hydrochemical investigations of groundwaters in the Kurihashi granodiorite at JNC's Kamaishi in-situ tests site indicate that these solutions are: (1)meteoric in origin, (2)chemically reducing (at depths greater than a few hundreds meters), (3)relatively young [residence times in the Kurihashi granodiorite generally less than about 40 years, but groundwaters older than several thousand years BP (before present) are also indicated by preliminary carbon-14 dating of samples obtained from the KH-1 borehole], (4)Ca-HCO type solutions near the surface, changing to Na-HCO
type solutions near the surface, changing to Na-HCO type groundwaters with increasing depth. The evolution of groundwater compositions in the Kurihashi granodiorite is modeled assuming local equilibrium for selected mineral-fluid reactions, taking into account the rainwater origin of these solutions. Results suggest it is possible to interpret approximately the "real" groundwater chemistry (i.e., pH, Eh, total dissolved concentrations of Si, Na, Ca, K, AI, carbonate and sulfate) in the Kurihashi granodiorite if the following assumptions are adopted: (1)CO
type groundwaters with increasing depth. The evolution of groundwater compositions in the Kurihashi granodiorite is modeled assuming local equilibrium for selected mineral-fluid reactions, taking into account the rainwater origin of these solutions. Results suggest it is possible to interpret approximately the "real" groundwater chemistry (i.e., pH, Eh, total dissolved concentrations of Si, Na, Ca, K, AI, carbonate and sulfate) in the Kurihashi granodiorite if the following assumptions are adopted: (1)CO concentration in the gas phase contacting pore solutions in the overlying soil zone = 10
concentration in the gas phase contacting pore solutions in the overlying soil zone = 10 bar, (2)minerals in the rock zone that control the solubility of respective elements in the groundwater include; chalcedony (Si), albite (Na), kaolinite (Al), calcite (Ca and carbonate), microcline (K) and pyrite (Eh and sulfate). Discussions with international experts suggest a systematic approach utilizing reaction-path models of irreversible water-rock interactions in open systems may be needed to more realistically model groundwater evolution at the Kamaishi test site. Detailed information characterizing certain site properties (e.g., fracture mineralogy) may be required to adequately constrain such models, however.
bar, (2)minerals in the rock zone that control the solubility of respective elements in the groundwater include; chalcedony (Si), albite (Na), kaolinite (Al), calcite (Ca and carbonate), microcline (K) and pyrite (Eh and sulfate). Discussions with international experts suggest a systematic approach utilizing reaction-path models of irreversible water-rock interactions in open systems may be needed to more realistically model groundwater evolution at the Kamaishi test site. Detailed information characterizing certain site properties (e.g., fracture mineralogy) may be required to adequately constrain such models, however.

 and AnO
and AnO
 Species in Geologic Environments
Species in Geologic EnvironmentsChoppin, G. R.*; Bronikowski, M.*; Chen, J.*; Byegard, J.*; Rai, D.*; Yui, Mikazu
JNC TN8400 99-012, 155 Pages, 1999/01
This report provides thermodynamic data for predicting concentrations of pentavalent and hexavalent actinide species (AnO and AnO
and AnO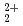 ) in geologic environments, and contributes to an integration of the JNC chemical thermodynamic database, JNC-TDB (previously PNC-TDB), for the performance analysis of geological isolation system for high-level radioactive wastes. Thermodynamic data for the formation of complexes or compounds with hydroxide, chloride, fluoride, carbonate, nitrate, sulfate and phosphate are discussed in this report. The estimation of the stability constants by use of the Born equation is included. The Pitzer parameters for AnO
) in geologic environments, and contributes to an integration of the JNC chemical thermodynamic database, JNC-TDB (previously PNC-TDB), for the performance analysis of geological isolation system for high-level radioactive wastes. Thermodynamic data for the formation of complexes or compounds with hydroxide, chloride, fluoride, carbonate, nitrate, sulfate and phosphate are discussed in this report. The estimation of the stability constants by use of the Born equation is included. The Pitzer parameters for AnO and AnO
and AnO
 , redox potentials and equilibrium constants of redox reactions for actinides are also included.
, redox potentials and equilibrium constants of redox reactions for actinides are also included.
Takano, Masahide; Minato, Kazuo; Fukuda, Kosaku; Sato, Seichi*; Ohashi, Hiroshi*
JAERI-Research 97-021, 19 Pages, 1997/03
no abstracts in English
; ;
Journal of Nuclear Materials, 151, p.63 - 71, 1987/00
Times Cited Count:10 Percentile:70.03(Materials Science, Multidisciplinary)no abstracts in English
 -'MC
-'MC '-C-CO; The system ZrO
'-C-CO; The system ZrO -'ZrC'-C-CO
-'ZrC'-C-COJ.Chem.Eng.Data, 27(2), p.186 - 188, 1982/00
Times Cited Count:4 Percentile:54.93(Thermodynamics)no abstracts in English
Journal of Solid State Chemistry, 36, p.305 - 313, 1981/00
Times Cited Count:0 Percentile:0.02(Chemistry, Inorganic & Nuclear)no abstracts in English
Miwa, Shuhei; Yamashita, Shinichiro; Osaka, Masahiko
no journal, ,
The effects of temperature and atmosphere on cesium (Cs) chemical form just after release from fuel were estimated using chemical equilibrium calculation with consideration of the release kinetics of Cs, iodine (I), boron (B) and so on depending on temperature and atmosphere under a severe accident. The calculation results show that Cs vapor species changed according to temperature and atmosphere, and CsBO vapor species was dominant with the existence of B regardless of temperature and atmosphere.
vapor species was dominant with the existence of B regardless of temperature and atmosphere.
 C control material on FP chemistry with VICTORIA code
C control material on FP chemistry with VICTORIA codeShiotsu, Hiroyuki; Ishikawa, Jun; Maruyama, Yu
no journal, ,
no abstracts in English
 and SiC in LWR conditions
and SiC in LWR conditionsShirasu, Noriko; Kurata, Masaki
no journal, ,
After Fukushima Daiichi nuclear accident in 2011, enhancing the accident tolerance of light water reactor fuels became a very important issue and currently the development of accident-tolerant fuel (ATF) are in progress. SiC is an attractive candidate of ATF cladding material because of its high chemical stability, high radiation resistance and low neutron absorption. SiC reacts much less than Zircaloy with steam, the generation of heat and hydrogen gas would be extremely suppressed. Thermodynamic evaluation on chemical interactions between UO and SiC were performed on various possible conditions of oxygen potential and temperature in severe accident. SiC is converted to SiO
and SiC were performed on various possible conditions of oxygen potential and temperature in severe accident. SiC is converted to SiO by reaction with O
by reaction with O . SiC is also converted to SiO
. SiC is also converted to SiO by reaction with H
by reaction with H O. However, the fraction of the sub-reaction for forming SiO increases than in the case of reaction with O
O. However, the fraction of the sub-reaction for forming SiO increases than in the case of reaction with O when comparing the results at the same temperatures and the same oxygen potentials.
when comparing the results at the same temperatures and the same oxygen potentials.